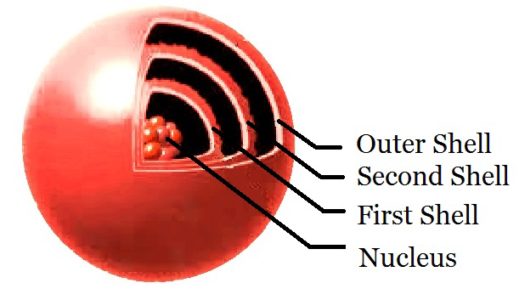
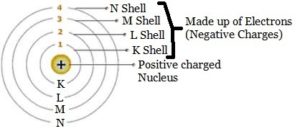
I will start from the very beginning with a little atomic theory. I will explain how it relates to basic-electronics concepts. Although atoms are made up of a number of smaller particles, only three are of primary interest for our purposes. The neutron, proton, and electron are the basic building blocks of the atom. Danish physicist Niels Bohr in 1913 came up with a theoretical model of the atom. The Boar model explains the theory behind static electricity, magnetic, current flow, voltage, and semiconductors. His model defines the center of the atom as the nucleus comprised of neutrons and protons. Orbiting the nucleus are electrons. The electrons are structured to form shells around the nucleus as pictured.
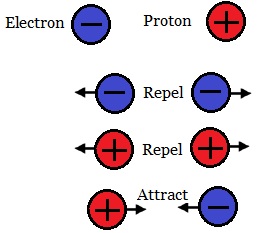
The particles exhibit a force between each other. They attract or repel other particles. The force is called charge. The charge of the Electron was arbitrarily assigned as negative and the charge of the proton as positive. The rule regarding these charges is that Like Charges repel each other and opposite charges attract each other.